Phoenix Fat Burner Supplement

Phoenix undefined
Fat Burner Supplement
$49.99
($0.56/capsule)1K+ bought last month
Get the only[1] fat burner supplement[2] with clinically effective doses[3] of 8 ingredients scientifically shown[4] to support fat loss and energy levels and reduce hunger and cravings . . . and without the jitters, upset stomach, or crash.[5]
- 30 peer-reviewed scientific studies support Phoenix’s combination of ingredients and doses[6]
- Contains no artificial fillers, food dyes, or other unnecessary junk[7]
- Tested for purity and potency in a state-of-the-art ISO 17025 accredited lab[8]
Will Phoenix melt fat from your belly, hips, and thighs faster than a sneeze in a cyclone?
No.
Will it supercharge your metabolism and energy levels?
Absolutely not.
But will Phoenix help you burn more calories and control your appetite without any harsh stimulants?
Yes. Or your money back.
- Total formulation transparency (no proprietary blends)[9]
- Made in the USA with globally sourced ingredients in NSF-certified and FDA-inspected facilities that adhere to Current Good Manufacturing Practice standards
- Backed by our “No Return Necessary” money-back guarantee that works like this: If you don’t absolutely love Phoenix, just let us know, and we’ll give you a full refund on the spot. No forms or returns necessary.
So order now, try Phoenix risk free, and see for yourself why we believe it’s the perfect fat burner supplement (and why it has sold over 250,000 bottles and counting!).
Will Phoenix melt fat from your belly, hips, and thighs faster than a sneeze in a cyclone?
No.
Will it supercharge your metabolism and energy levels?
Absolutely not.
But is Phoenix the only[1] fat burner supplement with clinically effective doses[2] of 8 ingredients[3] scientifically shown[4] to support fat loss and energy levels and reduce hunger and cravings . . . and without the jitters, upset stomach, or crash?[5]
And will it help you burn more calories and fat and control your appetite and cravings without any harsh stimulants?
Yes. Or your money back.
- 30 peer-reviewed scientific studies support Phoenix’s combination of ingredients and doses[6]
- Tested for purity and potency in a state-of-the-art ISO 17025 accredited lab[7]
- Contains no artificial fillers, food dyes, or other unnecessary junk[8]
- Total formulation transparency (no proprietary blends)[9]
- Made in the USA with globally sourced ingredients in NSF-certified and FDA-inspected facilities that adhere to Current Good Manufacturing Practice standards
Phoenix is also backed by our “No Return Necessary” money-back guarantee that works like this:
If you don’t absolutely love Phoenix, just let us know, and we’ll give you a full refund on the spot. No forms or returns necessary.
So order now, try Phoenix risk free, and see for yourself why we believe it’s the perfect fat burner supplement (and why it has sold over 250,000 bottles and counting!).
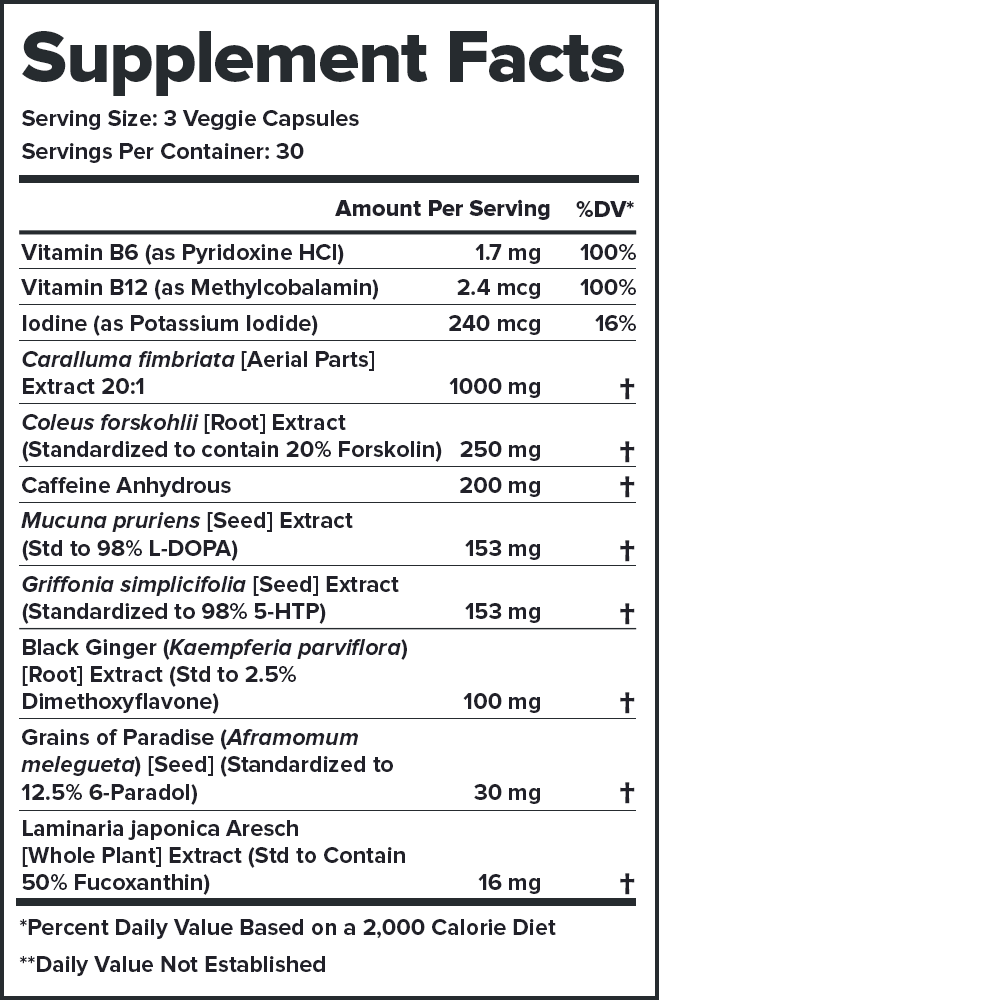
Legion Phoenix Ingredients (1,690 milligrams per serving)
See how Legion Phoenix compares to the rest.
- Active Ingredients
- Clinically Effective Ingredients
& Doses - Caffeine
- Black Ginger
- Grains of Paradise
- Forskolin
- Third-Party Lab Tested
- Labdoor Certified Brand
- Price Per Serving
The #1 brand of naturally sweetened sports supplements.
We’ve sold over 5 million bags and bottles to over 1 million customers in 169 countries who have left us over 45,000 5-star reviews.
Clinically Effective Ingredients and Doses
Every ingredient, form, and dose in Phoenix is backed by peer-reviewed scientific research demonstrating clear benefits in healthy humans.
No Unnecessary Junk
Phoenix contains no artificial food dyes, fillers, or other unnecessary junk.
Total Label Transparency
We clearly list the dose of each ingredient in Phoenix on the label—no proprietary blends or hidden ingredients—so you can verify our formulation’s validity and effectiveness.
Third-Party Lab Tested for Purity and Potency
Phoenix is tested by a state-of-the-art ISO 17025-accredited third-party laboratory for heavy metals, microbes, allergens, and other contaminants to ensure compliance with FDA purity standards.
Made in the USA
Phoenix is made in America with globally sourced ingredients in NSF-certified, FDA-inspected facilities that adhere to Current Good Manufacturing Practice (cGMP) standards.
"No Return Necessary"
Money-Back Guarantee
If you don't absolutely love Phoenix, you get a prompt and courteous refund. No forms or returns necessary.
Frequently Asked Questions
+References
Some popular fat burners are all-natural. Some contain the right mix of high-quality ingredients. Some provide clinically effective doses. But only Phoenix checks each of these boxes. ↑
Phoenix doesn’t just “contain natural ingredients''—every ingredient is naturally sourced from plants and animals. Phoenix contains no artificial or synthetic substances of any kind.↑
Every serving of Phoenix contains 1,690 milligrams of active ingredients that have been shown to be safe and effective in peer-reviewed scientific research.↑
Each active ingredient in Phoenix is backed by published scientific studies that show benefits in healthy humans.↑
Phoenix doesn’t contain any harsh stimulants that wind you up and burn you out—just caffeine, and just 200 milligrams per serving.↑
That’s 216 pages of scientific research that shows Phoenix works exactly like we say it does.↑
While these types of chemicals may not be as dangerous as some people claim, studies suggest that regular consumption of them may indeed be harmful to our health. And that’s why you won’t find them in Phoenix.↑
Every bottle of Phoenix is guaranteed to provide exactly what the label claims and nothing else—no heavy metals, microbes, allergens, or other contaminants.↑
This means you know exactly what’s in every serving of Phoenix—every dose of every ingredient—and can verify the accuracy and efficacy of the formulation.↑
Spriet LL. Sports Med. 2014;44 Suppl 2(Suppl 2):S175-S184. doi:10.1007/s40279-014-0257-8. ↑
McLellan TM, Caldwell JA, Lieberman HR. Neurosci Biobehav Rev. 2016;71:294-312. doi:10.1016/j.neubiorev.2016.09.001. ↑
Crawford C, Teo L, Lafferty L, et al. Nutr Rev. 2017;75(suppl_2):17-35. doi:10.1093/nutrit/nux007. ↑
Kamimori GH, McLellan TM, Tate CM, Voss DM, Niro P, Lieberman HR. Psychopharmacology (Berl). 2015;232(12):2031-2042. doi:10.1007/s00213-014-3834-5. ↑
Astrup A, Toubro S, Cannon S, Hein P, Breum L, Madsen J. Am J Clin Nutr. 1990;51(5):759-767. doi:10.1093/ajcn/51.5.759. ↑
Beck TW, Housh TJ, Schmidt RJ, Johnson GO, Housh DJ, Coburn JW, Malek MH. Department of Nutrition and Health Sciences, Human Performance Laboratory, University of Nebraska-Lincoln. J Strength Cond Res. 2006 Aug;20(3):506-10. ↑
Warren GL, Park ND, Maresca RD, McKibans KI, Millard-Stafford ML. Med Sci Sports Exerc. 2010;42(7):1375-1387. doi:10.1249/MSS.0b013e3181cabbd8.↑
Grgic J, Pickering C. J Sci Med Sport. 2019;22(3):353-360. doi:10.1016/j.jsams.2018.08.016.↑
Grgic J, Trexler ET, Lazinica B, Pedisic Z. J Int Soc Sports Nutr. 2018;15:11. Published 2018 Mar 5. doi:10.1186/s12970-018-0216-0.↑
Grgic J. Eur J Sport Sci. 2018;18(2):219-225. doi:10.1080/17461391.2017.1394371.↑
Beck TW, Housh TJ, Schmidt RJ, Johnson GO, Housh DJ, Coburn JW, Malek MH. Department of Nutrition and Health Sciences, Human Performance Laboratory, University of Nebraska-Lincoln. J Strength Cond Res. 2006 Aug;20(3):506-10. ↑
Warren GL, Park ND, Maresca RD, McKibans KI, Millard-Stafford ML. Med Sci Sports Exerc. 2010;42(7):1375-1387. doi:10.1249/MSS.0b013e3181cabbd8.↑
Polito MD, Souza DB, Casonatto J, Farinatti P. Sci Sports. 2016;31(3):119-128. doi:10.1016/J.SCISPO.2016.01.006.↑
Beck TW, Housh TJ, Schmidt RJ, Johnson GO, Housh DJ, Coburn JW, Malek MH. Department of Nutrition and Health Sciences, Human Performance Laboratory, University of Nebraska-Lincoln. J Strength Cond Res. 2006 Aug;20(3):506-10. ↑
Ganio MS, Klau JF, Casa DJ, Armstrong LE, Maresh CM. J Strength Cond Res. 2009;23(1):315-324. doi:10.1519/JSC.0b013e31818b979a.↑
Shen JG, Brooks MB, Cincotta J, Manjourides JD. J Sci Med Sport. 2019;22(2):232-238. doi:10.1016/j.jsams.2018.07.022.↑
Southward K, Rutherfurd-Markwick KJ, Ali A. [published correction appears in Sports Med. 2018 Aug 9;:]. Sports Med. 2018;48(8):1913-1928. doi:10.1007/s40279-018-0939-8.↑
Desbrow B, Biddulph C, Devlin B, Grant GD, Anoopkumar-Dukie S, Leveritt MD. J Sports Sci. 2012;30(2):115-120. doi:10.1080/02640414.2011.632431.↑
Ganio MS, Klau JF, Casa DJ, Armstrong LE, Maresh CM. J Strength Cond Res. 2009;23(1):315-324. doi:10.1519/JSC.0b013e31818b979a.↑
McLellan TM, Caldwell JA, Lieberman HR. Neurosci Biobehav Rev. 2016;71:294-312. doi:10.1016/J.NEUBIOREV.2016.09.001. ↑
Guest NS, VanDusseldorp TA, Nelson MT, et al. J Int Soc Sports Nutr. 2021;18(1). doi:10.1186/S12970-020-00383-4. ↑
Temkitthawon P, Hinds TR, Beavo JA, Viyoch J, Suwanborirux K, Pongamornkul W, Sawasdee P, Ingkaninan K. J Ethnopharmacol. 2011 Oct 11;137(3):1437-41. doi: 10.1016/j.jep.2011.08.025. ↑
Yoshino S, Kim M, Awa R, Kuwahara H, Kano Y, Kawada T. Food Sci Nutr. 2014 Nov;2(6):634-7. doi: 10.1002/fsn3.144. ↑
Okabe Y, Shimada T, Horikawa T, Kinoshita K, Koyama K, Ichinose K, Aburada M, Takahashi K. Phytomedicine. 2014 May 15;21(6):800-6. doi: 10.1016/j.phymed.2014.01.014. ↑
Matsushita M, Yoneshiro T, Aita S, Kamiya T, Kusaba N, Yamaguchi K, Takagaki K, Kameya T, Sugie H, Saito M. J Nutr Sci Vitaminol (Tokyo). 2015;61(1):79-83. doi: 10.3177/jnsv.61.79. ↑
Stein RA, Schmid K, Bolivar J, Swick AG, Joyal SV, Hirsh SP. J Integr Med. 2018 Jul;16(4):249-254. doi: 10.1016/j.joim.2018.05.005. ↑
Promthep K, Eungpinichpong W, Sripanidkulchai B, Chatchawan U. Med Sci Monit Basic Res. 2015 May 6;21:100-8. doi: 10.12659/MSMBR.894301. ↑
Wattanathorn J, Muchimapura S, Tong-Un T, Saenghong N, Thukhum-Mee W, Sripanidkulchai B. Evid Based Complement Alternat Med. 2012;2012:732816. doi: 10.1155/2012/732816. ↑
Sugita J, Yoneshiro T, Sugishima Y, Ikemoto T, Uchiwa H, Suzuki I, Saito M. J Nutr Sci Vitaminol (Tokyo). 2014;60(1):22-7. doi: 10.3177/jnsv.60.22. ↑
Sugita J, Yoneshiro T, Hatano T, Aita S, Ikemoto T, Uchiwa H, Iwanaga T, Kameya T, Kawai Y, Saito M. Br J Nutr. 2013 Aug;110(4):733-8. doi: 10.1017/S0007114512005715. ↑
Litosch I, Hudson TH, Mills I, Li SY, Fain JN. Mol Pharmacol. 1982 Jul;22(1):109-15. ↑
Pinto C, Papa D, Hübner M, Mou TC, Lushington GH, Seifert R. J Pharmacol Exp Ther. 2008 Apr;325(1):27-36. doi: 10.1124/jpet.107.131904. ↑
Godard MP, Johnson BA, Richmond SR. Obes Res. 2005 Aug;13(8):1335-43. doi: 10.1038/oby.2005.162. ↑
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Biochem Biophys Res Commun. 2005 Jul 1;332(2):392-7. doi: 10.1016/j.bbrc.2005.05.002. ↑
Abidov M, Ramazanov Z, Seifulla R, Grachev S. Diabetes Obes Metab. 2010 Jan;12(1):72-81. doi: 10.1111/j.1463-1326.2009.01132.x. ↑
Kang SI, Ko HC, Shin HS, Kim HM, Hong YS, Lee NH, Kim SJ. Biochem Biophys Res Commun. 2011 Jun 17;409(4):769-74. doi: 10.1016/j.bbrc.2011.05.086. ↑
Maeda H, Hosokawa M, Sashima T, Murakami-Funayama K, Miyashita K. Mol Med Rep. 2009 Nov-Dec;2(6):897-902. doi: 10.3892/mmr_00000189. ↑
Kuriyan R, Raj T, Srinivas SK, Vaz M, Rajendran R, Kurpad AV. Appetite. 2007 May;48(3):338-44. doi: 10.1016/j.appet.2006.09.013. ↑
Kell G, Rao A, Katsikitis M. J Affect Disord. 2019 Mar 1;246:619-626. doi: 10.1016/j.jad.2018.12.062. ↑
Birdsall TC. Altern Med Rev. 1998 Aug;3(4):271-80. ↑
Ceci F, Cangiano C, Cairella M, Cascino A, Del Ben M, Muscaritoli M, Sibilia L, Rossi Fanelli F. J Neural Transm. 1989;76(2):109-17. doi: 10.1007/BF01578751. ↑
Wurtman RJ, Wurtman JJ. Obes Res. 1995 Nov;3 Suppl 4:477S-480S. doi: 10.1002/j.1550-8528.1995.tb00215.x. ↑
Perez-Cornago A, Ramírez MJ, Zulet MÁ, Martinez JA. Psychoneuroendocrinology. 2014 Sep;47:98-106. doi: 10.1016/j.psyneuen.2014.05.003. ↑
Gardner DF, Centor RM, Utiger RD. Clin Endocrinol (Oxf). 1988 Mar;28(3):283-8. doi: 10.1111/j.1365-2265.1988.tb01214.x. ↑
Chow CC, Phillips DI, Lazarus JH, Parkes AB. Clin Endocrinol (Oxf). 1991 May;34(5):413-6. doi: 10.1111/j.1365-2265.1991.tb00314.x. ↑
Majchrzak D, Singer I, Männer M, Rust P, Genser D, Wagner KH, Elmadfa I. Ann Nutr Metab. 2006;50(6):485-91. doi: 10.1159/000095828. ↑
Naik S, Mahalle N, Greibe E, Ostenfeld MS, Heegaard CW, Nexo E, Fedosov SN. Nutrients. 2019 Oct 6;11(10):2382. doi: 10.3390/nu11102382. ↑